Background: TP53 mutations, found in around 20% of AML cases, often lead to therapy resistance and unfavorable outcomes. Recent studies hint at the potential efficacy of CD47, a ‘don't eat me’ signal, in treating TP53-mutated AML. The ongoing clinical trial (NCT04435691) is assessing the efficacy of a triple therapy combo-magrolimab (anti-CD47 mAb), azacitidine, and venetoclax (AVM)-with promising initial results in TP53-mutated AML patients. However, understanding resistance and relapse remains crucial.
Methods: We performed paired 5' scRNA and scTCR profiling on 3 healthy donors and 27 samples from 11 treatment-naive TP53-mutated AML patients (median age 63, range 59-73 years; 8 responders (CR/CRi); 3 non-responders (NR)), both pre-AVM (n=11) and post-AVM-point of remission (n=11), relapse (n=2), or primary resistance (n=3).
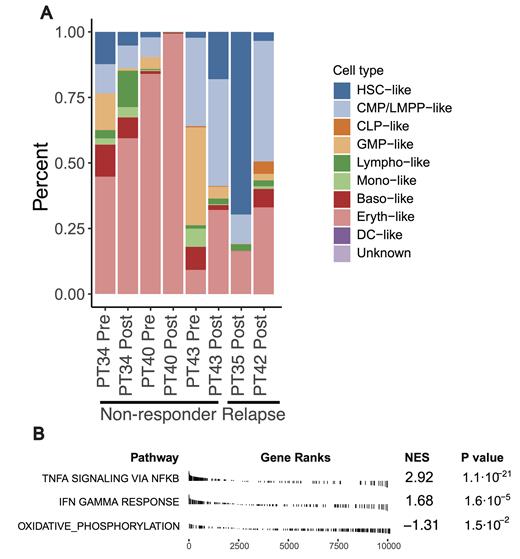
Results: 42,910 AML cells and 137,169 bone marrow microenvironment cells passed quality checks. AML cell identification was confirmed via correlation with clinical flow cytometry, inferred copy number alterations, and immunohistochemistry markers. We leveraged Symphony to phenotypically infer AML cell types based on their transcriptional similarity to normal hematopoietic cells. Overall, samples displayed heterogeneous distribution of cell types at time of diagnosis. However, the 3 samples from the 3 primary resistant patients demonstrated a marked and significant expansion of AML cell proportion exhibiting an erythroid differentiation phenotype post-AVM (Fig. A). Also, the 2 patients who relapsed 4.8- and 7.1-months following AVM demonstrated expansion of erythroid differentiation proportion (Fig. A) and higher LSC17 at time of relapse. This suggests a selection favoring erythroid clones as a mechanism of primary and acquired resistance following AVM therapy. We conducted a pathway enrichment analysis using Hallmark gene sets to identify significantly altered AML-intrinsic biological pathways between responders and non-responders. CR/CRi patient-derived AML cells exhibited an enriched inflammatory response, specifically in TNFα and IFNγ signaling pathways-known CD47 expression regulators-and increased CD47 expression (Fig. B). In contrast, AML cells from non-responsive patients showed an enrichment of oxidative phosphorylation (Fig. B). Of note, phagocytic activity of myeloid cells was high in non-responders suggesting that despite the likely activity of magrolimab, other mechanisms of primary resistance were at play.
As anti-CD47-mediated phagocytosis can stimulate antigen presentation and trigger T cell activation, we delved into the T cell subtypes and their corresponding cellular responses. There were not significant differences in the distribution of CD4 and CD8 pretreatment in CR/CRi versus NR patients. However, CD8 dysfunction score was significantly higher in non-responders at baseline. Following therapy, the CD8 dysfunction score was also significantly higher at time of relapse among responders. Further, effector CD8 T cells of NR patients had enrichment for cytotoxicity and TCR signaling pathways, and higher expression of exhaustion-related genes.
Conclusions: Through single cell transcriptomic profiling of TP53-mutated AML patients treated with the triplet combination of AVM on protocol we identified erythroid differentiation and oxidative phosphorylation as possible intrinsic resistance mechanisms. Additionally, non-responders exhibited a dysfunctional T cell state prior to treatment, while relapses were associated with development of CD8 dysfunction. Overall, this data provides valuable insight into the mechanisms of response and resistance to magrolimab-based therapy. TCR repertoire and AML-immune cell interaction analyses are ongoing.
Disclosures
Senapati:Kite Pharma: Other: Advisory Board. Pemmaraju:Dan's House of Hope: Membership on an entity's Board of Directors or advisory committees; Aplastic Anemia & MDS International Foundation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; PharmaEssentia: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Menarini Group: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Physician Education Resource (PER): Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; PeerView Institute for Medical Education: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Dava Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Harborside Press: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ASH Committee on Communications: Other: Leadership; ClearView Healthcare Partners: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Stemline: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pacylex: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Curio Science: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Karger Publishers: Other: Licenses; ASCO Cancer.Net Editorial Board: Other: Leadership; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; EUSA Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Protagonist Therapeutics, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Imedex: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; National Institute of Health/National Cancer Institute (NIH/NCI): Research Funding; Patient Power: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; OncLive: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Medscape: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Cimeio Therapeutics AG: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Neopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CTI BioPharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ImmunoGen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Intellisphere: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Magdalen Medical Publishing: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CancerNet: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CareDx: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; United States Department of Defense (DOD): Research Funding; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Blueprint: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Aptitude Health: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; HemOnc Times/Oncology Times: Other: Uncompensated; Bristol Myers Squibb Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Kadia:Janssen Research and Development: Research Funding; Glycomimetics: Research Funding; Hikma Pharmaceuticals: Speakers Bureau; Ascentage Pharma Group: Research Funding; Pulmotect, Inc.: Consultancy, Research Funding; Delta-Fly Pharma, Inc.: Research Funding; Pfizer: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; GenFleet Therapeutics: Research Funding; SELLAS Life Sciences Group: Research Funding; Liberum: Consultancy; Novartis: Consultancy; Cellenkos Inc.: Research Funding; Cure: Speakers Bureau; Regeneron Pharmaceuticals: Research Funding; Cyclacel: Research Funding; Iterion: Research Funding; Sanofi-Aventis: Consultancy; Celgene: Research Funding; AstraZeneca: Research Funding; Astellas Pharma Global Development: Research Funding; Amgen, Inc.: Research Funding; Jazz Pharmaceuticals, Pfizer, Pulmotect, Inc, Regeneron Pharmaceuticals, SELLAS Life Sciences Group: Research Funding; Genzyme: Honoraria; Biologix, Cure, Hikma Pharmaceuticals: Speakers Bureau; AbbVie, Amgen, Inc, Ascentage Pharma Group, Astellas Pharma Global Development, Astex, AstraZeneca, BMS, Celgene, Cellenkos Inc, Cyclacel, Delta-Fly Pharma, Inc, Genentech, Inc., Genfleet, Glycomimetics, Iterion, Janssen Research and Development: Research Funding; Agios: Consultancy; Daiichi Sankyo, Genentech, Inc., Genzyme, Jazz Pharmaceuticals, Liberum, Novartis, Pfizer, PinotBio, Inc, Pulmotect, Inc, Sanofi-Aventis, Servier: Consultancy; Servier: Consultancy; Pinotb-Bio: Consultancy; BMS: Consultancy, Research Funding; Astex: Honoraria. Ravandi:Astex/taiho: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene/BMS: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Xencor: Research Funding; Biomea fusion: Honoraria, Research Funding; Astellas: Consultancy, Honoraria, Research Funding; Syros: Consultancy, Honoraria, Research Funding; Prelude: Research Funding. Garcia-Manero:Genentech: Research Funding; Bristol Myers Squibb: Other: Medical writing support, Research Funding; AbbVie: Research Funding. Daver:AROG: Consultancy; Trillium: Consultancy, Research Funding; Trovagene: Research Funding; Hanmi: Research Funding; Novartis: Consultancy; Celgene: Consultancy; Genentech: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Shattuck Labs: Consultancy; ImmunoGen: Consultancy, Research Funding; Jazz: Consultancy; Glycomimetics: Research Funding; Gilead: Consultancy, Research Funding; Servier: Consultancy, Research Funding; Syndax: Consultancy; Agios: Consultancy; Astellas: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Kite, a Gilead company: Consultancy, Research Funding; FATE: Research Funding; Novimmune: Research Funding; Kronos Bio: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal